4 Which of the Following Compounds Is a Strong Electrolyte
CH 3 COOH acetic acid d. SrOH 2 - strontium hydroxide.

Strong And Weak Electrolytes Substances Whose Aqueous Solutions Produce Lots Of Free Moving Ions Are Called Strong Electrolytes Substances Whose Aqueous Ppt Download
NaC 2 H 3 O 2.
. Which of the following compounds is a strong electrolyte. Group of answer choices. HBr - hydrobromic acid.
Write the reaction when potassium carbonate is put into water. A sucrose b acetic acid c methyl alcohol d water e sodium hydroxide. KNO3 KNO2 HNO3 HNO2.
These chemicals completely dissociate into ions in aqueous solution. A H2O D CH3CH2OH ethanol B N2 E KOH C CH3COOH acetic acid. Therefore C is a correct answer.
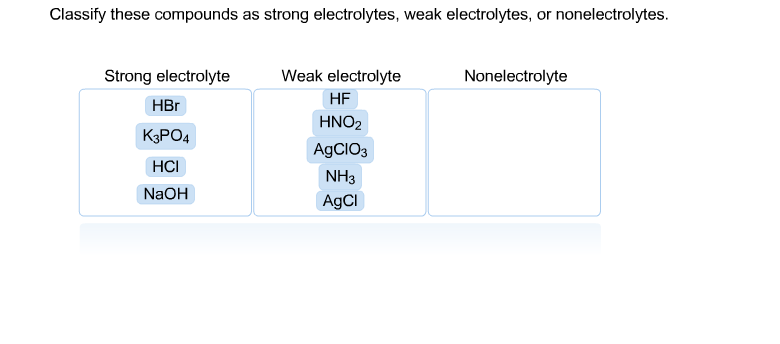
1 Which of the following compounds is a strong electrolyte. HNO3 is strong electrolyte and CH3COOH is weak electrolyte. Salts much have high solubility in the solvent to act as strong electrolytes.
There are virtually no molecules of a strong acid or base in solution only ions. HCO3- aq H20 aq. Identify the major ionic species present in an aqueous solution of K S04.
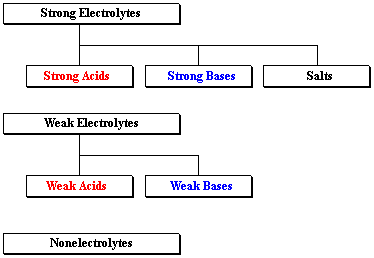
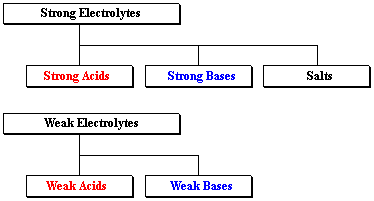
A HO B N C CHCOOH acetic acid D CH3CH2OH ethanol E KOH 12. HCl hydrochloric acid H 2 SO 4 sulfuric acid NaOH sodium hydroxide and KOH potassium hydroxide are all strong electrolytes. Strong electrolytes include the strong acids strong bases and salts.
This compound is molecular because it does not contain a metal cation nor an ammonium ion. Which of the following compounds is a weak electrolyte. HCl - hydrochloric acid.
Strong acids and strong bases are strong electrolytes eg HClaq H 2 SO 4 aq HClO 4 aq. What is the formula weight molar mass of a compound if 20 g of the compound in 500 mL of solution gives a 05 M solution. N H X 3 ce NH_3 NH X 3.
School Thomas Edison State College. A Chapter 4- Reactions in Aqueous Solutions - Chapter 4. View exam 2 sample questionspdf from CHM 211 at Notre Dame University-Louaize.
A HCl D O 2 B CH 3COOH acetic acid E NaCl C C 6H 12O. Hydrochloric acid sodium hydroxide aluminum chloride. The compound potassium carbonate is a strong electrolyte.
Identify which substance below is NOT a strong acid in aqueous solution. Identify the strong base in aqueous solution from among the following compounds. Which of the following compounds is a strong electrolyte.
View Chapter4Part1Practicepdf from CHM 1045 at Florida International University. Which of these compounds is a strong electrolyte Select one. Pages 26 Ratings 95 56 53 out of 56 people found this document helpful.
H 2 O b. Which of the following compounds is a strong electrolyte. Strong acids strong bases and ionic salts that are not weak acids or bases are strong electrolytes.
N a I ce NaI NaI. Isotonic solution is 5 glucose or 09 NaCl. CH3COOH acetic acid Answer.
Hydrogen chloride HCl strong electrolyte ions only 3. Course Title CHE 111. A H 2O D CH 3CH 2OH ethanol B N 2 E KOH C CH 3COOH acetic acid Ans.
Identify the following compound as an electrolyte or nonelectrolyte. Which of the following compounds is a strong electrolyte. Which of the following compounds is a strong electrolyte.
Chemistry 4th Edition Edit edition Solutions for Chapter 41 Problem 4CP. 7 NaCl 3 If the following reaction. A KS O B K2 S.
Which of the following compounds is a strong electrolyte. Hydrofluoric acid metyll amine. Group of answer.
Chapter 4 Part 1 Practice Student. Which of these compounds is a strong electrolyte. All of the above 2 A red blood cell will undergo crenation in _____.
0 D 2K S 402- E 2K SO2- 13. Net ionic equation for the following molecular equation. Which of the following compounds is a weak electrolyte.
Which of the following compounds is a strong electrolyte. Which of the following compounds is a strong electrolytea HFb H2CO3c NaFd NH3e H2O. So this compound is a strong electrolyte.
2Which of the following compounds is a strong electrolyte. Identify the following compounds as a strong electrolytes weak electrolytes or nonelectrolytes. C 2 H 6 O ethanol e.
See the answer See the answer done loading. A Na 2 S b CuOH 2 c NH 3. HI - hydroiodic acid.
NaOH - sodium hydroxide. The molarity M of a solution that contains 20 moles of glucose in 40 L of solution is_____. Which of the following compounds is a strong electrolyte.
This compound is ionic because it contains a metal cation and an anion. Which of the following substances is a strong electrolyte. NaCl - sodium chloride.
40- C 2K So. Know the strong acids and bases on the accompanying hand-out Strong and Weak Acids and Bases. The electrical conductivities of the following 0100 M solutions were measured in an apparatus that contained a light bulb as.
A HBr b HCl c HNO 3 d H 2 SO 4 e HF. CH3COOH acetic acid Answer.

What Are Some Examples Of A Strong Electrolyte Quora

Solved Classify These Compounds As Strong Electrolytes Weak Chegg Com
What S In A Name The Questions Asked On This Quiz Are Of Five Basic Forms What Is The Formula Of What Is The Name Of What Type Of Compound Is Which Of The Following Is A What Are The Principal Species In A Solution Of It Is Very Important For
Comments
Post a Comment